Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Miyamoto, Yutaka; Yasuda, Kenichiro; Magara, Masaaki
no journal, ,
Plutonium and Americum are artificial elements which originate from nuclear bomb tests and accidental releases from nuclear power plants. The authors accomplished the development of technique for sequential separation of U, Th, Pb, the lanthanides, Pu and Am using a single anion-exchange column and mixed acids. An automatic system assembled for this work sequentially separated the elements of interest.
Toh, Yosuke; Ebihara, Mitsuru*; Huang, M.; Kimura, Atsushi; Nakamura, Shoji; Segawa, Mariko
no journal, ,
no abstracts in English
Huang, M.; Toh, Yosuke; Ebihara, Mitsuru*; Kimura, Atsushi; Nakamura, Shoji
no journal, ,
 ssbauer spectroscopic parameters
ssbauer spectroscopic parametersKaneko, Masashi
no journal, ,
This study aims to perform the optimization of density functional methods using experimental data of M ssbauer isomer shifts and apply these methods to d, f-block coordination chemistry. In the d-block complexes, the validity for the spin-crossover (SCO) switching behavior of iron(II) assembled complexes was confirmed. The application of this method indicated that whether SCO occurs or not depends on the dihedral angle between iron atom and pyridine plane. In the f-block complexes, the benchmarking of computational method was performed using
ssbauer isomer shifts and apply these methods to d, f-block coordination chemistry. In the d-block complexes, the validity for the spin-crossover (SCO) switching behavior of iron(II) assembled complexes was confirmed. The application of this method indicated that whether SCO occurs or not depends on the dihedral angle between iron atom and pyridine plane. In the f-block complexes, the benchmarking of computational method was performed using 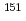 Eu and
Eu and  Np M
Np M ssbauer isomer shifts. The optimized method reproduced the separation behavior of Am(III) ion from Eu(III) ion and implied that the difference in the bonding contribution between 5f(Am) and 4f(Eu) attributes to the selectivity of Am(III) compared with Eu(III).
ssbauer isomer shifts. The optimized method reproduced the separation behavior of Am(III) ion from Eu(III) ion and implied that the difference in the bonding contribution between 5f(Am) and 4f(Eu) attributes to the selectivity of Am(III) compared with Eu(III).
Tsukada, Kazuaki; Hashimoto, Kazuyuki*; Hatsukawa, Yuichi*; Kawabata, Masako*; Saeki, Hideya*; Minato, Futoshi; Iwamoto, Nobuyuki; Nagai, Yasuki*; Sugo, Yumi*; Watanabe, Satoshi*; et al.
no journal, ,
no abstracts in English
Kaneko, Masashi; Watanabe, Masayuki; Miyashita, Sunao*; Nakashima, Satoru*
no journal, ,
To understand the separation mechanism of minor-actinides (MA) from lanthanides (Ln) is important for the development of partitioning and transmutation of high-level radioactive waste. We aim to discuss the bonding nature between MA/Ln ions and ligands with the separation behavior of MA from Ln, which leads to the elucidation of the selectivity of MA compared to Ln. In this study, we demonstrate the separation behavior of MA from Ln at molecular level by means of density functional calculations and discuss how the bonding properties of valence electrons contributes to the selectivity of MA.
Ishii, Yasuo; Mitachi, Katsuaki; Abe, Hironobu; Niizato, Tadafumi
no journal, ,
Mountainous forest is currently one of the most important sources of radioactive cesium released from the Fukushima Daiichi Nuclear Power Plant Accident in Fukushima. This study reports the changes in amounts and concentrations of radioactive cesium of the surface run-off substances and dose rate at forest observation plots in Ogi district of Kawauchi-mura and Yamakiya district of Kawamata-machi, and Ogaki soil saving dam, Fukushima during 2013 - 2015.
Tomita, Jumpei; Miyata, Yoshiki*; Hama, Katsuhiro; Sakaguchi, Aya*; Nagao, Seiya*; Yamamoto, Masayoshi*
no journal, ,
no abstracts in English
Kitatsuji, Yoshihiro; Ouchi, Kazuki; Otobe, Haruyoshi
no journal, ,
Redox behaviors of actinide ions in a low-acid solution are complicated and have been unclear, because they tend to form hydroxide complexes, colloids and precipitates. Recently, autocatalysis of the reduction of U(V) to U(IV) in the solution where U(IV) forms hydroxide colloid was reported; U(IV) colloid formed as reduction product catalyzes the electrolytic reduction of U(V). In this study, possibility of catalysis of Zr(IV) colloid which was analogous of U(IV) colloid on the reduction of U(V) was investigated. When U(VI) was electrolyzed at a constant potential in order to reduce to U(V) in the solution of pH 3.0 containing 10 mM Zr(IV), the increase of the reduction current was observed in initial stage of electrolysis. This results indicates that U(VI) was reduced further to U(IV) without catalysis of U(IV) colloid, and that Zr(IV) colloid also acts as catalyst. Intensity of catalysis depends on concentration of Zr(IV), condition of preparing colloid and acidity of the solution.
Fujita, Hiroki; Nojima, Takehiro*; Nagaoka, Mika; Osawa, Takahito; Ono, Hironobu*
no journal, ,
no abstracts in English
Satou, Yukihiko; Sueki, Keisuke*; Sasa, Kimikazu*
no journal, ,
no abstracts in English
Tomita, Ryohei*; Matsunaka, Tetsuya*; Honda, Maki*; Satou, Yukihiko; Matsumura, Masumi*; Takahashi, Tsutomu*; Sakaguchi, Aya*; Matsuzaki, Hiroyuki*; Sasa, Kimikazu*; Sueki, Keisuke*
no journal, ,
no abstracts in English
Yasuda, Kenichiro; Suzuki, Daisuke; Kanazawa, Kazuhito; Miyamoto, Yutaka; Esaka, Fumitaka; Magara, Masaaki
no journal, ,
We are applying a screening technique with alpha-ray measurement to the analysis of particles containing nuclear materials in swipe samples. In the present study, individual plutonium particles with known purification dates prepared from a standard reference material (NBS SRM947) were measured with alpha-ray spectrometry. The obtained ( Am+
Am+ Pu)/
Pu)/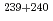 Pu activity ratios for the particles with the diameters of less than 2 micrometers were consistent with the reference values within the uncertainties. These results indicate that alpha spectrometry can be applied to the quantitative analysis of
Pu activity ratios for the particles with the diameters of less than 2 micrometers were consistent with the reference values within the uncertainties. These results indicate that alpha spectrometry can be applied to the quantitative analysis of  Am in individual plutonium particles.
Am in individual plutonium particles.
Toyoshima, Atsushi; Kanda, Akimitsu*; Ikeda, Takumi*; Yoshimura, Takashi*; Shinohara, Atsushi*; Yano, Shinya*; Komori, Yukiko*; Haba, Hiromitsu*
no journal, ,
Astatine (At) is reported to be bound in a few oxidation states in aqueous solutions. However, their valencies and chemical species are experimentally not identified. In this study, we studied redox behavior of At using redox agents and using a flow electrolytic column.  At was produced in the
At was produced in the 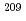 Bi(
Bi( , 2
, 2 )
) At reaction and was then separated from the target by a distillation method. Then, redox of At was carried out in 1.0 M HClO
At reaction and was then separated from the target by a distillation method. Then, redox of At was carried out in 1.0 M HClO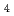 using redox agents or by an electrolysis. After the redox, solvent extraction with HDEHP was performed to identify the redox reactions. Using the redox agents, extraction yields of At were different between the conditions with oxidizing or reducing agents and without redox agents. This means that redox reactions of At can be identified under the present extraction conditions. On the other hand, by the electrolysis, extraction yields of At were almost constant against the variation of applied potential. This is possibly due to the restoration to the original oxidation state after the electrolysis. In the future, we will perform flow electrolytic column chromatography available for simultaneous electrolysis and separation.
using redox agents or by an electrolysis. After the redox, solvent extraction with HDEHP was performed to identify the redox reactions. Using the redox agents, extraction yields of At were different between the conditions with oxidizing or reducing agents and without redox agents. This means that redox reactions of At can be identified under the present extraction conditions. On the other hand, by the electrolysis, extraction yields of At were almost constant against the variation of applied potential. This is possibly due to the restoration to the original oxidation state after the electrolysis. In the future, we will perform flow electrolytic column chromatography available for simultaneous electrolysis and separation.
Sato, Tetsuya; Kaneya, Yusuke*; Asai, Masato; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina*; Osa, Akihiko; Makii, Hiroyuki; Nishio, Katsuhisa; Hirose, Kentaro; et al.
no journal, ,
The ground state electronic configuration of lawrencium (Lr, Z =103) is predicted to be [Rn]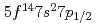 , which is different from that of the lanthanide homolog Lu [Xe]
, which is different from that of the lanthanide homolog Lu [Xe]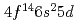 due to strong relativistic effects. According to semi-empirical considerations, volatility of Lr is expected to be higher than that of Lu. We have investigated adsorption behavior of
due to strong relativistic effects. According to semi-empirical considerations, volatility of Lr is expected to be higher than that of Lu. We have investigated adsorption behavior of  Lr, which was produced in the reaction of
Lr, which was produced in the reaction of  Cf(
Cf(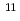 B, 4n), on a tantalum (Ta) metal surface using a surface ion-source installed into the isotope separator on-line (ISOL) at the JAEA tandem accelerator facility. The observed adsorption behavior of
B, 4n), on a tantalum (Ta) metal surface using a surface ion-source installed into the isotope separator on-line (ISOL) at the JAEA tandem accelerator facility. The observed adsorption behavior of  Lr was similar to those of Tb and Lu which have relatively higher adsorption enthalpy on Ta surface. It implies that Lr would have low volatility like such as Lu and Tb.
Lr was similar to those of Tb and Lu which have relatively higher adsorption enthalpy on Ta surface. It implies that Lr would have low volatility like such as Lu and Tb.
Sato, Tetsuya
no journal, ,
The first ionization potential (IP ) of element 103, lawrencium (Lr), has been successfully determined for the first time by using a newly developed method based on a surface ionization process. The measured IP
) of element 103, lawrencium (Lr), has been successfully determined for the first time by using a newly developed method based on a surface ionization process. The measured IP value is 4.96
value is 4.96  0.08 eV. This value is the smallest among those of actinide elements and is in excellent agreement with the value of 4.963(15) eV predicted by state-of-the-art relativistic calculations also performed in this work. Our results strongly support that the Lr atom has an electronic configuration of [Rn]
0.08 eV. This value is the smallest among those of actinide elements and is in excellent agreement with the value of 4.963(15) eV predicted by state-of-the-art relativistic calculations also performed in this work. Our results strongly support that the Lr atom has an electronic configuration of [Rn]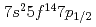 , which is influenced by strong relativistic effects. The present work opens the way for studies on atomic properties of heavy elements with atomic number
, which is influenced by strong relativistic effects. The present work opens the way for studies on atomic properties of heavy elements with atomic number 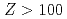 . Moreover, the present achievement has triggered a controversy on the position of lutetium (Lu) and Lr in the Periodic Table of Elements.
. Moreover, the present achievement has triggered a controversy on the position of lutetium (Lu) and Lr in the Periodic Table of Elements.